Green hydrogen is frequently regarded as a ‘fuel of the future’ – It has many benefits including its use in a wide variety of industries, its substantial energy storage capacity and most importantly, it produces zero emissions.
There is a hydrogen rainbow which exists to categorise the different hydrogen production processes. The most common hydrogen production types within industry include Grey, Blue and Green Hydrogen;
- Grey Hydrogen is produced through steam methane reforming and releases carbon dioxide into the atmosphere.
- Blue Hydrogen is also produced through steam methane reforming; however, it has a carbon capture and storage step to vastly reduce the carbon emissions released into the atmosphere.
- Green Hydrogen is produced by water separation through electrolysis powered by renewable energy sources, such as wind or solar. No carbon emissions are produced in this process.
There are several different electrolyser technologies available for the separation of water into hydrogen and oxygen:
- PEM electrolysers (Proton Exchange Membrane) – Water splits into oxygen, hydrogen protons and electrons. The hydrogen protons exchange across the membrane and the electrons flow through an external circuit. The hydrogen protons and the electrons recombine to form neutral hydrogen, which has been separated from the oxygen.
- Alkaline electrolysers – They consist of 2 metallic electrodes, a microporous separator, and an aqueous alkaline solution, typically 30% Potassium Hydroxide. Water splits into hydrogen and hydroxide ions; the hydroxide ions can move across the separator whereas hydrogen ions cannot, therefore separating it from the oxygen.
- AEM electrolysers (Anion Exchange Membrane) – Anion exchange membranes have positively charged functional groups. It offers the efficiency and reliability of PEM without the scarce metal electrocatalysts.
- SOEC (Solid Oxide Electrolyser Cell) – Working at temperatures above 500°C, utilising the high temperatures to make hydrogen from steam and waste process heat.
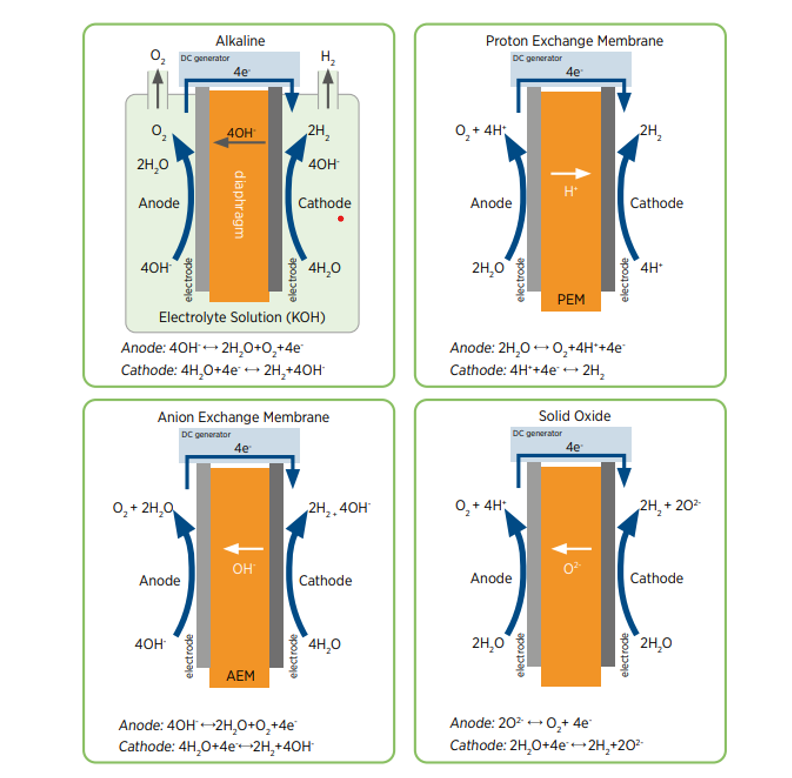
The Alkaline and PEM electrolysers are the most mature and commercially available technologies for production of Green Hydrogen. The main differences between the two technologies are outlined below:
| Alkaline Electrolyser | PEM Electrolyser |
| •No scarce metals required for the electrocatalyst. | •Scarce metals required for electrocatalyst e.g. Iridium, Platinum or Ruthenium |
| •Cannot easily respond to renewable energy fluctuations in electricity feed. | •Capable of handling renewable energy fluctuations i.e., fast ramp up time |
| •Potential for corrosive KOH electrolyte leaks | •No corrosive electrolyte involved in the process. |
| •Typically, hydrogen leaves the process at lower purity and pressures than PEM electrolysers. | •Typically, produces better hydrogen gas purity and higher pressures than alkaline electrolysers. |
| •Typically, the process is less efficient than PEM electrolysis. | •Typically, PEM is a more efficient process than alkaline electrolysis. |
In summary, Alkaline and PEM electrolysers are the most accepted green hydrogen production technologies currently available. Both electrolysers have different benefits and drawbacks depending on their project parameters, and neither technology can provide the perfect solution to green hydrogen production, however, both have their place in industry and are helping us move towards the reality of making hydrogen our ‘fuel of the future’.
For further information on the Hydrogen rainbow, please see our previous blog – The current Hydrogen Energy Sector.


